Create a Medical Device Certificate Application (CFG)Step-by-Step Instructions
April, 2016
Return to Online Registration1
Table of Contents
- Create a Medical Device Certificate Application
- Navigation
- Section 1 Requestor Information
- Section 2 Manufacturer Information
- Section 3 Distributor Information (if applicable)
- Section 4 Product Information
- Section 5 Was the product ever recalled?
- Section 6 List country(ies) for which the Certificates are requested
- Section 7 Indicate what product information should appear on the certificate
- Section 8 Should the country destination be listed on the certificate?
- Section 9 Exporter’s Certification Statement
- Review Screen
Create a CFG Application
Log into the FDA Industry Systems and select 'CDRH Export Certification Application & Tracking System' from the list of systems available on the FURLS Home Page as shown in Figure 1.
The CECATS Main Menu page as shown in Figure 2 below :-
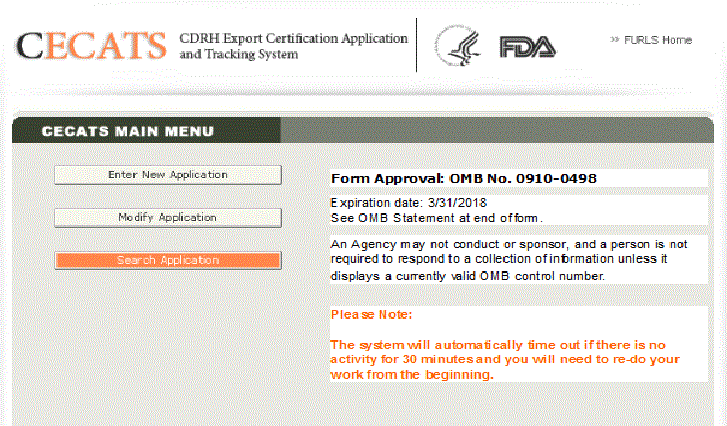
To create a new application, click Enter New Application. . All applications that you have saved or submitted will be displayed as shown in Figure 3 below. Applications that are saved, but not submitted will be in “Draft” status until you submit them.
- To create a new application, click "Enter New Application". Then skip to the first paragraph after Figure 3 to continue.
- To continue working on a draft application, click the desired application radio button and click "Complete Draft Application".
- To clone a previous application, click the desired application radio button and click "Clone Application". Verify the information on each page is still correct and continue through all screens.
Click Modify to makes changes to a submitted, but not yet in Under Review status.
The following options are available:
- Modify application based on a notification received
- Change the number of certs
- Upload corrected Exporter’s Certification Statement (ECS)
- Cancel request
To search using the application number, click Search Application. This option may be used to continue working on a draft application, to clone a previous application, or to make corrections to an application that is in 'Return for Action' status.
NOTE: Return for Action status indicates that upon review of the application by the FDA, additional information or clarification was required. CECATS will send an email to the requestor with comments from the reviewer and will provide a 48 hour window for modifications to be made. If modifications cannot be made within 48 hours, simply clone the application when ready, modify and submit. A new application number will be assigned. No charges are incurred for an application unless certificates are issued.
NOTE: For all applications in a 'Draft' status, if you do not perform any activity within 30 days, the system will automatically change the status to 'Not Submitted'. Please clone to continue the application.
The Center for Devices and Radiological Health (CDRH) issues several types of Export Certificates and letters. When creating a new application, select which application type to be requested as shown in Figure 4.
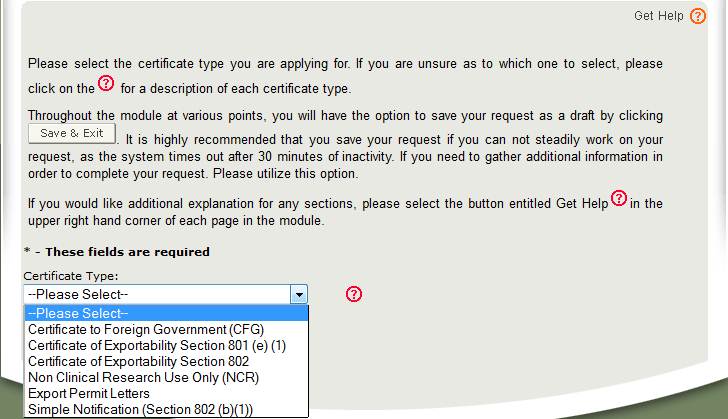
Select Certificate to Foreign Government (CFG).
Description of each Application Type:
For a description of each application type, select the red question mark icon located next to the Application Type dropdown list shown in Figure 4 above.
Certificate to Foreign Government (CFG)
A Certificate to Foreign Government is issued for legally marketed devices in the United States that are in compliance with the requirements of the Federal Food, Drug, and Cosmetic Act (FD&C).
Eligibility Note:
- Are cleared or approved by FDA for marketing in the U.S.
- May be legally commercially distributed in the U.S.
- May be a Class I, II, or III device
- Are listed with the FDA
- All devices must be exported from the United States
Acceptable CFG Marketing Statuses: 510(k), PMA, Exempt, Pre-amendment, Humanitarian Device Exemption (HDE), or Product Development Protocol (PDP).
Certificate of Exportability 801(e)(1)
Medical devices that are not FDA approved or cleared for marketing legally in the U.S. may be exported under section 801(e)(1) of the FD&C Act, provided that they are intended for export only. Although such devices do not meet the requirements of the FD&C Act to be sold in the U.S., they may be exported legally and without FDA permission per section 801(e)(1) if they are class I or class II devices and they are:
- In accordance with the specifications of the foreign purchaser;
- Not in conflict with the laws of the country to which they are intended for export;
- Labeled on the outside of the shipping package that they are intended for export; and
- Not sold or distributed in the U.S.
In addition, the U.S. Exporter must comply with the laws of the importing country.
Certificate of Exportability Section 802
The Certificate of Exportability Section 802 is for the export of products not approved for marketing in the United States that meet the requirements of Section 801(e)(1) and Section 802 of the Federal Food, Drug, and Cosmetic Act. Among the requirements to be met prior to the issuance of this certificate are the following:
- Class II and III devices, and class I devices associated with class III.
- Devices are not approved or cleared by FDA for marketing in the U.S.,
- Manufacturing establishments meet Good Manufacturing Practices (GMP) regulations and
- The manufacturer must be registered with FDA.
The manufacturing facility must be in compliance with the registration requirements.
The device must be in compliance with the listing requirements
The device must meet the requirements of Section 801(e):
- accords to the specifications of the foreign purchaser.
- is not in conflict with the laws of the country to which it is intended for export.
- is labeled on the outside of the shipping package that it is intended for export.
- is not sold or offered for sale in domestic commerce.
In addition, the U.S. Exporter must comply with the laws of the importing country.
Non-Clinical Research Use Only Certificate
The Non-Clinical Research Use Only Certificate is for the export of a non-clinical research use only product, material, or component that is not intended for human use which may be marketed in, and legally exported from the United States under the Federal Food, Drug, and Cosmetic Act. Among the requirements to be met prior to the issuance of this certificate are the following:
- The "Non-Clinical Research Use Only" certificate is for product(s), material(s), or component(s) that are not used to prevent, treat, or diagnose human disease.
- The manufacturing facility is required to label these products according to 21 CFR 809.10(c)(2)(i) or 21 CFR 312.160, as appropriate.
- All products listed on Non-Clinical Research Use Only Certificate must be exported from the U.S.
- Each Non-Clinical Research Use Only Certificate request must be requested by the U.S. manufacturer. Requests received from a foreign firm will not be considered. A U.S. firm must appear on each Non-Clinical Research Use Only Certificate.
- All contract manufacturers and contract sterilizers involved in the manufacturing process must be identified on the 3613c form regardless if they are to appear on the certificate.
Export Permit Letter
To obtain an Export Permit Letter, which affirms the FDA's approval to export devices in accordance with section 801(e)(2) of the FD&C Act, a request should include the following information:
- A complete description of the device intended for export;
- The status of the device in the U.S., e.g., whether it is investigational, banned, etc.,
- A statement indicating that the requestor conducted a search of the Medical Literature Analysis and Retrieval System database (see instructions below) and a summary of the search results, as well as a summary of safety data to demonstrate that export of the device will not endanger the public health and safety, or documentation of exemption from this requirement (see below), and
- A letter from the appropriate foreign liaison (person with authority to sign a letter of acceptance for the foreign government) identified in the CDRH Foreign Liaison Listing12, which must be either in English or accompanied by a certified English translation, stating that:
- The device is not in conflict with the laws of the country to which it is intended for export;
- The foreign government has full knowledge of the status of the device in the U.S.; and
- Import is permitted or not objected to
Simple Notification
While the FDA does not require a firm to obtain written permission prior to export under section 802, firms exporting a device under section 802 must provide written notification to the FDA.
The notification must identify:
- The product's trade name;
- The type of device;
- The product's model number; and
- The country that is to receive the exported product if the export is to a "not listed" country (non-Tier 1 country).
If the export is intended for a "listed" Tier 1 country, then the notification may, but is not required to, identify the importing country. Or it may state that export is to a Tier 1 country without identifying the listed country.
Navigation
A status bar at the top of every page will track your progress through each step of the application process as shown in Figure 6 below.
A "Get Help" icon, located at the top right of each page, will provide page specific help. For an overview of all help files available, please refer to the FDA Industry Systems Index of Help Pages at https://www.access.fda.gov/cecats/help/CreateApplication.html.
Also located the top right corner of each page is a "FURLS HOME" link that will take you to the FURLS Home Page. The "CECATS HOME" link will take you to the CECATS Home Page (Refer to Figure 1). To log out of the system, select "FURLS HOME" and click on logout.
At the top and bottom of each screen are navigation buttons as shown in Figure 6 below.
- Back -Navigates back one screen or back one step. Information entered on the current screen will NOT be saved if you select the "Back" button.
- Save & Exit– Information entered up to this point will be saved. Your application will be set to a "Draft" status until you complete and submit the application. If you do not perform any activity for 30 days, the system will automatically cancel and delete the application. When you log into the CECATS system, all applications in a "Draft" status will be displayed after selecting the "Enter New Application" option from the main menu.
- Save & Continue– Information entered up to this point will be saved in 'Draft' status. The system will generate the application number when you click on the 'Save & Continue' button the first time and this number shall be visible on the top of the screen until the application is submitted. Please keep the Application number for your records and for any communications with FDA regarding this application. Your application will be set to a “Draft” status until you complete and submit the application. If you do not perform any activity for 30 days, the system will automatically change the status to 'Not Submitted' and may delete the application.
- Continue to Step X - All information is saved on the current screen and navigates to the beginning of the next step. However, exiting CECATS without using the "Save & Exit" button will cause the loss of the entire request.
- Cancel & Start Again- The system will return you to the screen where you selected the Application Type. All information you have entered will NOT be saved.
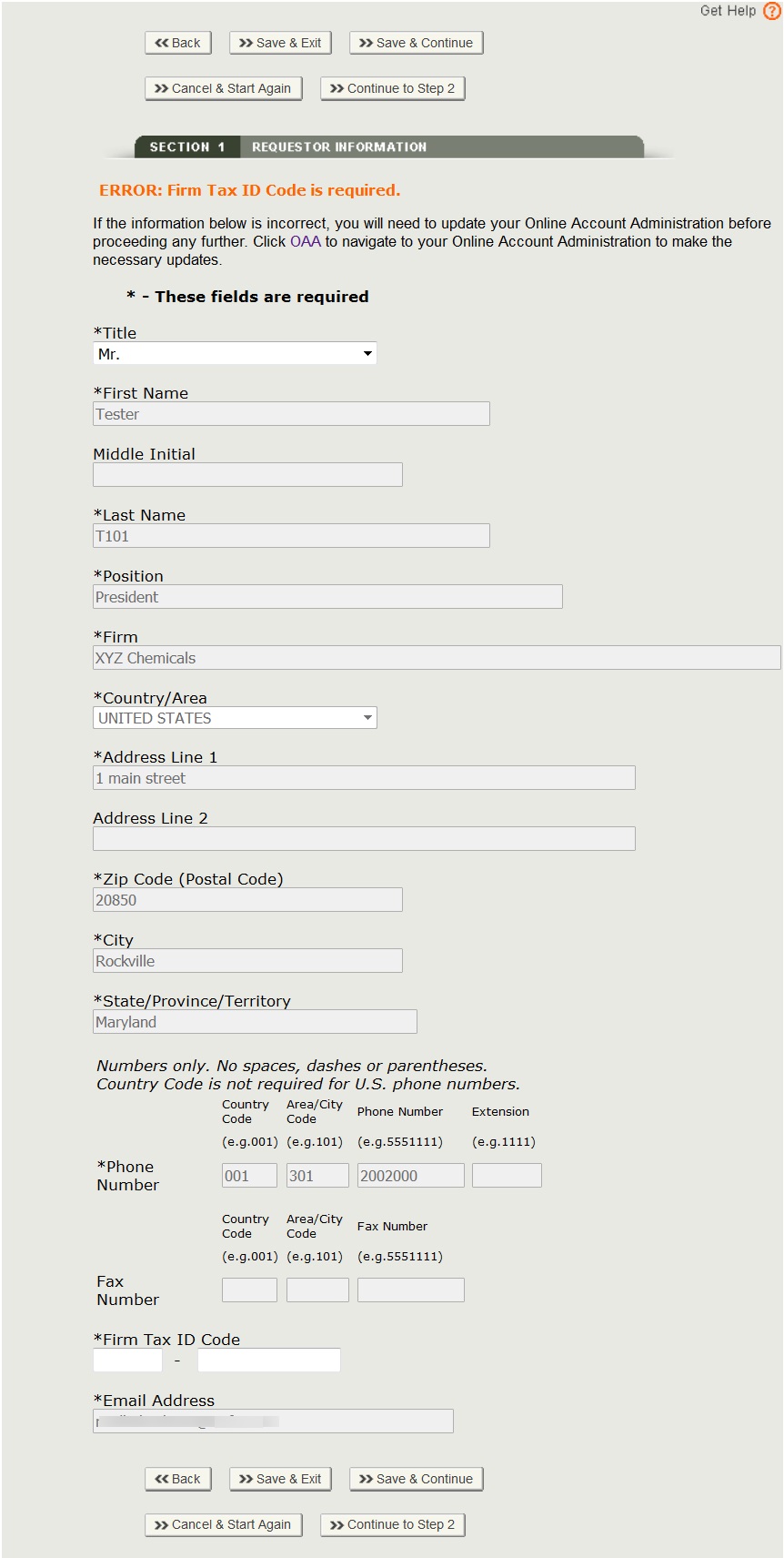
NOTE: Fields marked with an asterisk (*) are mandatory fields. You will NOT be able to proceed to the next step or Save & Exit until all mandatory fields have been properly entered on the current screen.
Section 1 - Requestor Information
The system auto-populates specific information from your Online Account Administration (OAA) into section 1 (Requestor Information) of the application. These fields cannot be edited in CECATS.
If the information is incorrect, click the 'FURLS Home' link as shown in Figure 6. Then click "Edit Account Profile" on the left-hand side and update your account profile accordingly.
The following two fields are required in section 1:
- Title
- Firm Tax ID Code (also referred to as the Employer Identification Number or EIN (a nine-digit numeric value). This number is assigned by the Internal Revenue Service (IRS).
Click “Continue to Step 2”. See Figure 7 below.
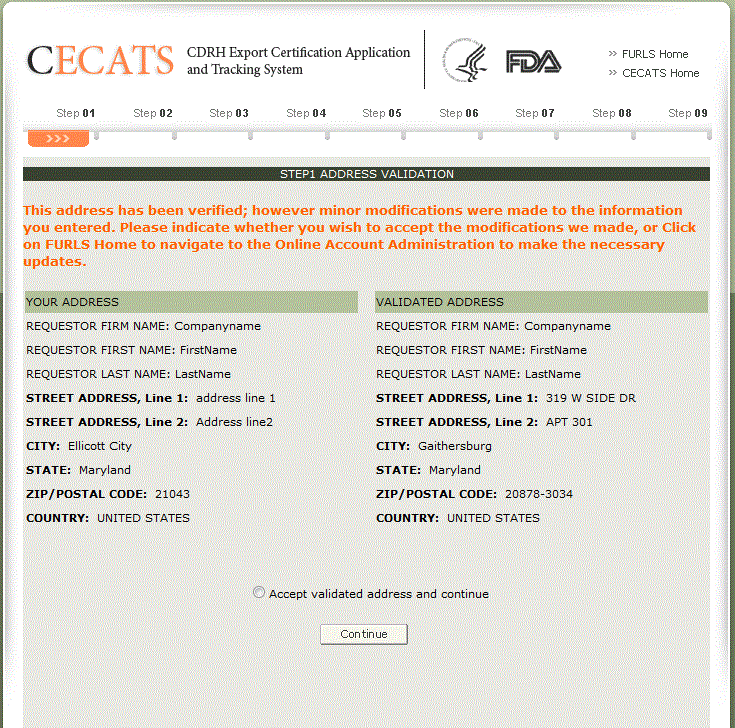
At the next screen, you will need to validate your address. This address is the requestor’s address. It is not used as the facility address on the application or for shipping. See Figure 9 below.
Click "Accept validated address and continue" and click "Continue" to proceed to Step 2. See Figure 8 below.
Section 2 - Manufacturer Information
The FDA requires that all facilities manufacturing medical devices must be registered in the FDA Device, Registration and Listing Module (DRLM). Once a manufacturer is registered in DRLM, the FDA provides an Owner Operator Number to the requesting firm. The FDA also assigns a Registration Number for every manufacturer registered in DRLM. If you have not registered your manufacturer in DRLM, return to the FURLS home page and register your manufacturer in DRLM. Otherwise, please enter the Registration Number or the Owner Operator Number.
NOTE: In this section, you must identify all Manufacturers, Specification Developers, Repackagers/Relabelers, Remanufacturers, Reprocessor of Single Use Devices, Foreign Exporters, Contract Manufacturers, and Contract Sterilizers that are involved in the manufacturing process of devices on this request.
Additionally, if you include a Specification Developer then you must include the Contract Manufacturer for products on this request. Conversely, if you include a Contract Manufacturer, then the Specification Developer must also be included.
Enter the Registration Number or the Owner Operator Number (OON) and click on the “Retrieve Registration” button. See Figure 9.

Owner Operator number (OON) entered:
As long as the OON is active, the system will display all facilities associated with the OON. Select one or more facilities (if applicable) from the list of manufacturers that are in active status as shown in Figure 10 below. Inactive facilities will be grayed out and cannot be selected.
Registration number entered:
Only the single facility associated with that number will be displayed.
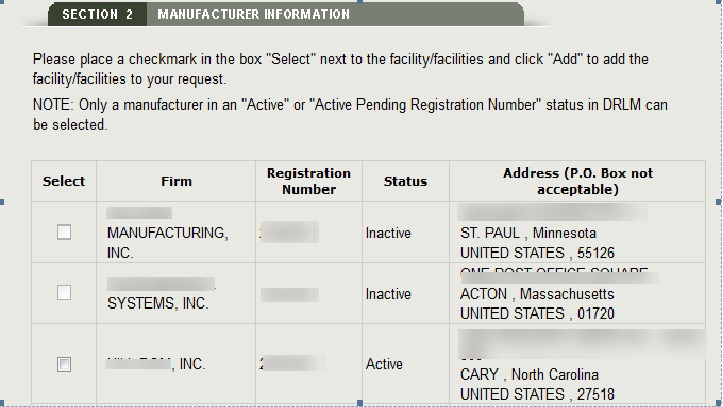
- A facility may be entered only one time in section 2.
- After selecting one or more manufacturing facilities associated with your Owner Operator number, you have the option to add one or more facilities associated with a different Owner Operator Number.
After selecting the manufacturer(s), a review page for all manufacturers selected as shown in Figure 11.
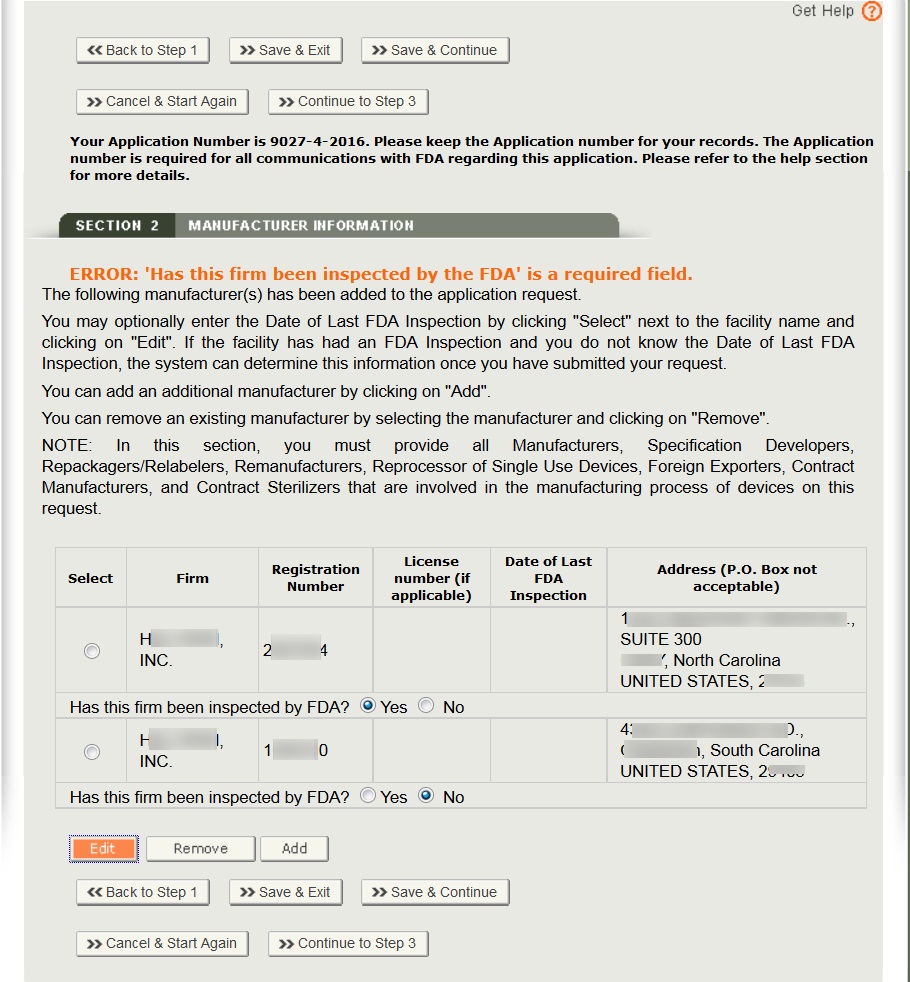
Select the radio button to answer “Has this firm been inspected by FDA?”.
To add additional manufacturer(s), click “Add”.
To remove a manufacturer from the list, select the facility, click on “Remove”, and verify by selecting the “Continue” button.
Optional: To enter the date of last FDA inspection for each manufacturer, select the facility, click “Edit” and enter the Date of Last FDA Inspection. See Figure 12 below.
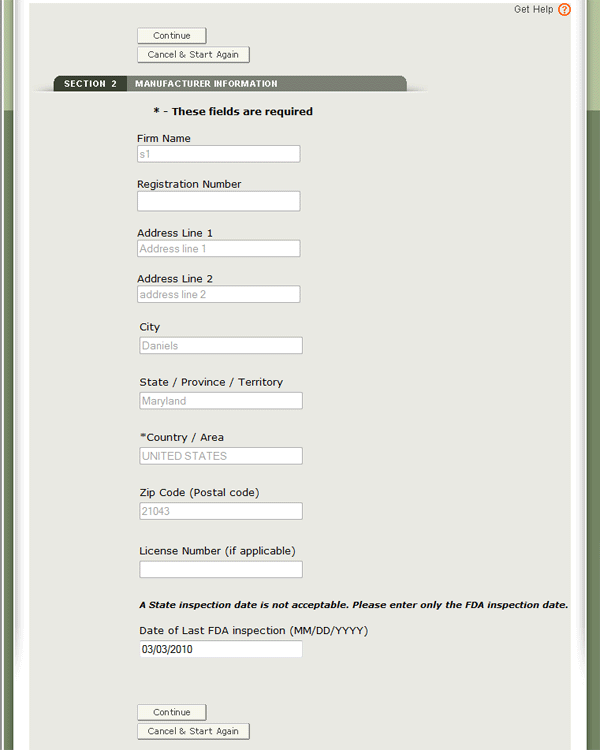
Once you have completed selecting all manufacturers, click “Continue to Step 3”.
Section 3 - Distributor Information
In section 3, you have the option to enter one or more Distributors to your request. This is the U.S. establishment that exports the devices from the United States. Do not include foreign distributors.
Enter the Registration Number or the Owner Operator Number (OON) and click on “Retrieve Registrations”. See Figure 13 below.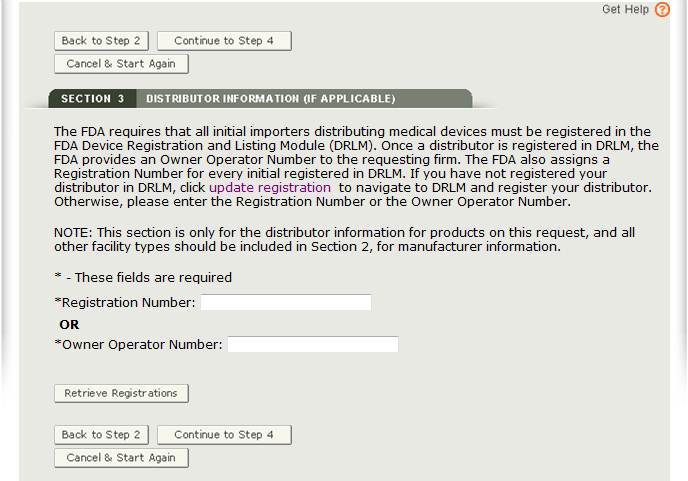
Similar to the section 2, the establishment information will appear. If correct, click the facility and click “Add”. If not correct, click “Back”. See Figure 14 below. Multiple distributors can be added.
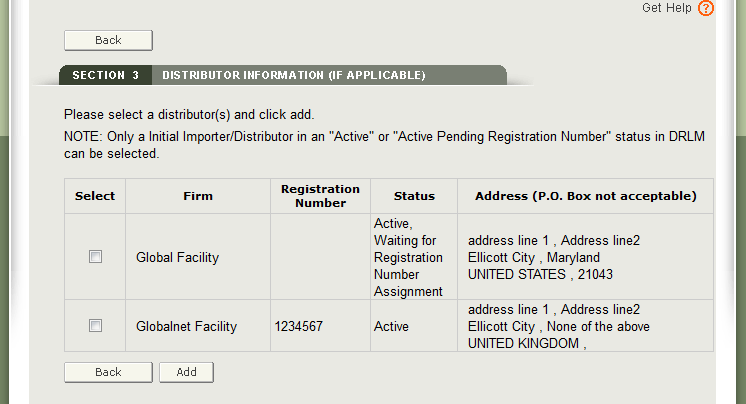
- Only facilities in an active DRLM status can be chosen. An inactive facility will be grayed out.
- A facility may be entered one time only in section 3.
- NOTE: All distributors must have a U.S. Address. If you do not add a distributor in section 3, at least one manufacturer must have a U.S. Address. You will not be able to proceed to section 4 unless one manufacturer or distributor has a domestic address.
To add another distributor, click “Add”
To remove a distributor from the list, select the facility, click “Remove”, and verify by clicking the “Continue” button.
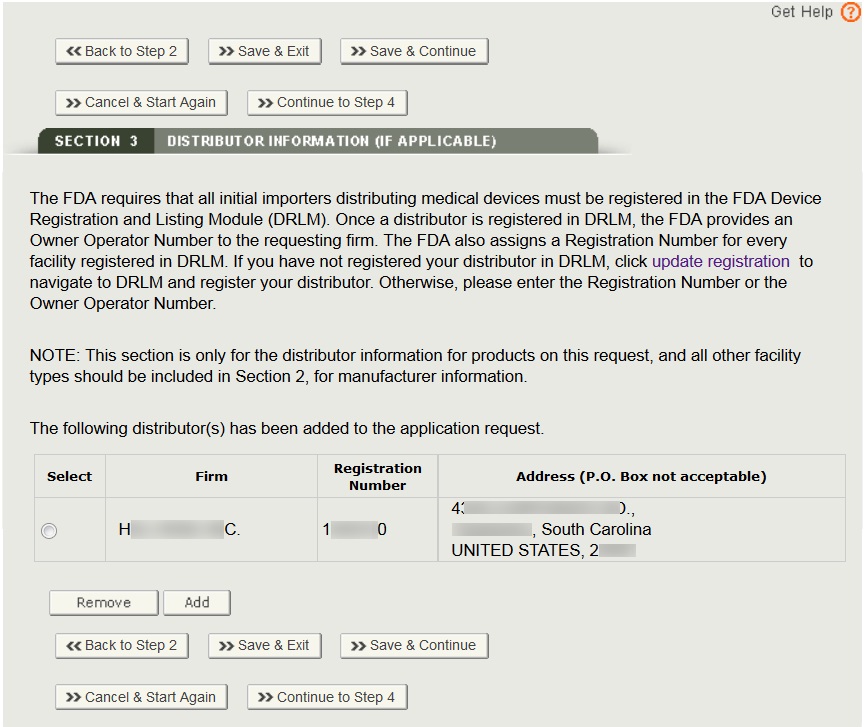
After all distributors have been identified, click on “Continue to Step 4”.
After selecting the distributor(s), the system will display a review page of all distributors selected as shown in Figure 15.
Section 4 –Product Information
In this section, you will be able to select one or more products from each manufacturer identified in sections 2.
- Class III products may be included only if all of the manufacturing facility(ies) have been inspected by the FDA. Failure to comply will prompt the FDA to immediately reject the certificate request application.
- Refurbished or remanufactured product may be listed on this application if the facility you represent is the original manufacturer. Additionally, the products must be identified as refurbished or remanufactured on the certificate.
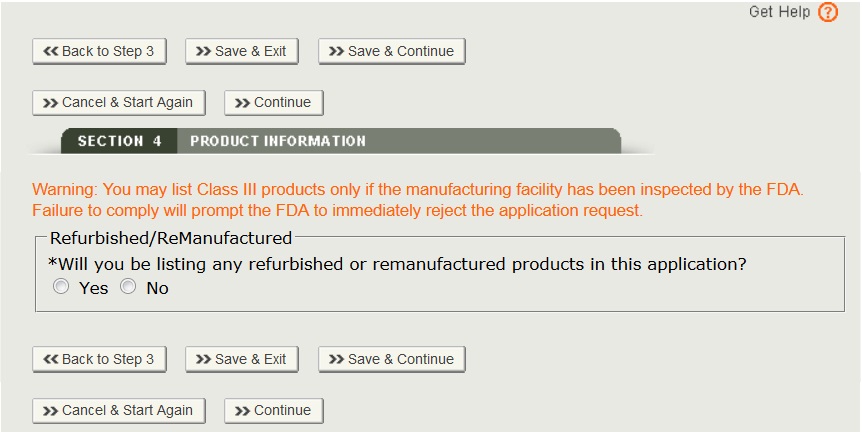
Select “Yes” or “No” to the question(s) regarding refurbished or remanufactured products have been answered, a list of the manufacturers and distributors will be displayed as shown in Figure 17 below.
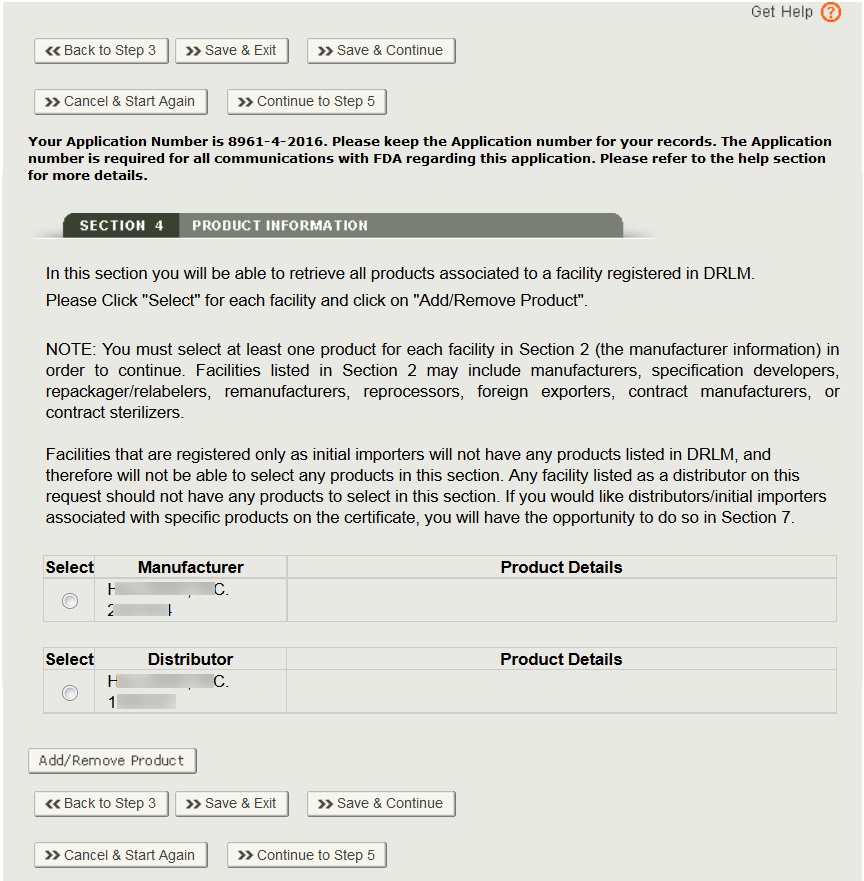
NOTE: You must add at least one product for each manufacturer.
Add Products
To add a product, first select the manufacturer and click on the Add/Remove Product button. The system will display the product listing for that manufacturer (from DRLM). Select the radio button corresponding to the product(s) you wish to add. Once you have selected the checkbox, you will also be able to enter the Proper Name as shown in Figure 18 below.
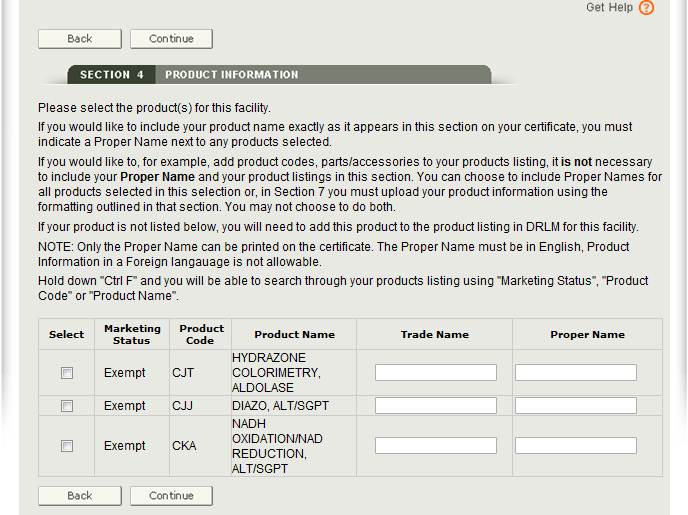
IMPORTANT: Please select all the products for this facility that are to be included on the certificate. If any product is not listed on the facility’s establishment registration, the facility will need to update their DRLM registration before you can continue.
At this point, you have an option regarding the addition of the product name. You can either enter the name of the devices exactly as you want it to appear on the certificate in the Proper Name block OR you can wait until section 7 and upload a product list. If you want to include product codes, parts/accessories to the product listing, do not indicate a Proper Name in this section. Instead, in section 7, upload the product information using the formatting outlined in that section.
To avoid the device name appearing on the certificate more than once, list the Proper Name of each device only once even if multiple firms have listings for the same device.
To remove a product from a facility, select the facility and click “Add/Remove Product”. Deselect the corresponding radio button and click “Continue.
NOTE: You are not required to add a product to a distributor.
Once all products have been selected, click “Continue to Step 5”.
Section 5 – Was the product ever recalled?
All recalls (both open and closed) that have occurred within the past ten years for any product on the application must be identified.
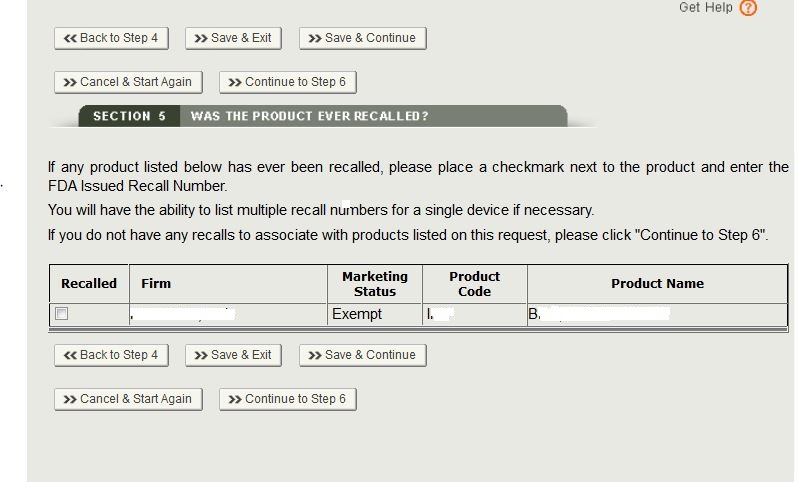
Select the checkbox next to the product A new button will appear. Click on the “Add/
Modify Recall Info” button as shown in Figure 19a.
Enter the following Recall Number information: • FDA Issued Recall Number • Close-out Date • Internal Reference Number, if no FDA recall number has been issued yet.
NOTE: The format of the recall number is Z-XXXX-YYYY. Please enter the eight-digit number as shown in Figure 19b below.
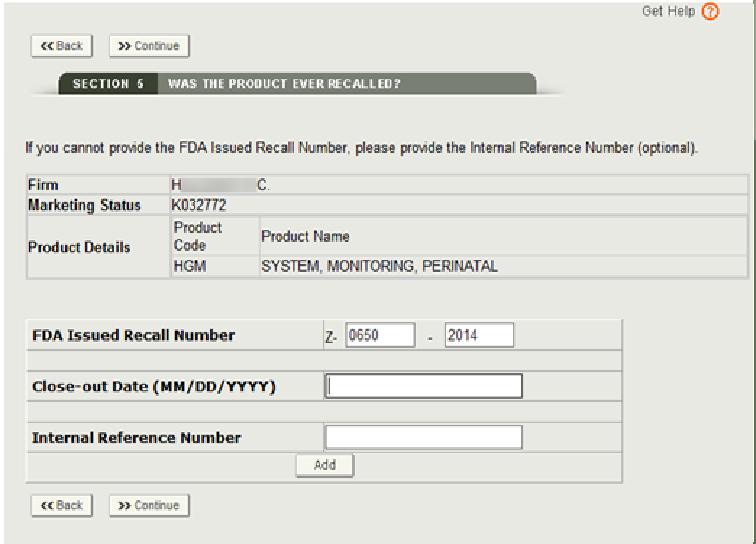
Click “Continue” and the system will validate the recall number. If the number is valid, the Close-out Date field may get auto-populated.
If no Close-out Date was found for the recalled product, an option to upload the official Close-out Letter (from the FDA) will be provided. Please do not upload your firm’s letter to the FDA. The accepted file formats include the following: pdf, bmp, jpeg, gif, png, tiff, doc, and docx.
To upload the FDA Close-out letter, click Browse… and navigate to the file on your computer as shown in Figure 20 below
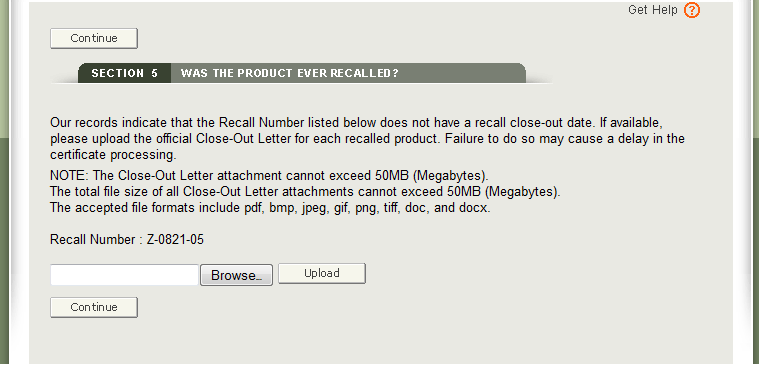
Select the file and click “Upload”.. The system will display the link as shown in Figure 21 below. To remove the attachment, simply click “Remove”.

Section 6 – List Country(ies) for which the Certificates are requested
Select one or more countries to indicate the product destination, click “Add” and repeat for additional countries as shown in Figure 22.
NOTE: Another method to select a country (other than scrolling down the list) is to first click on a country from the country list and then type in the first few letters of the desired country name. The system will jump to the country that begins with the letters typed.
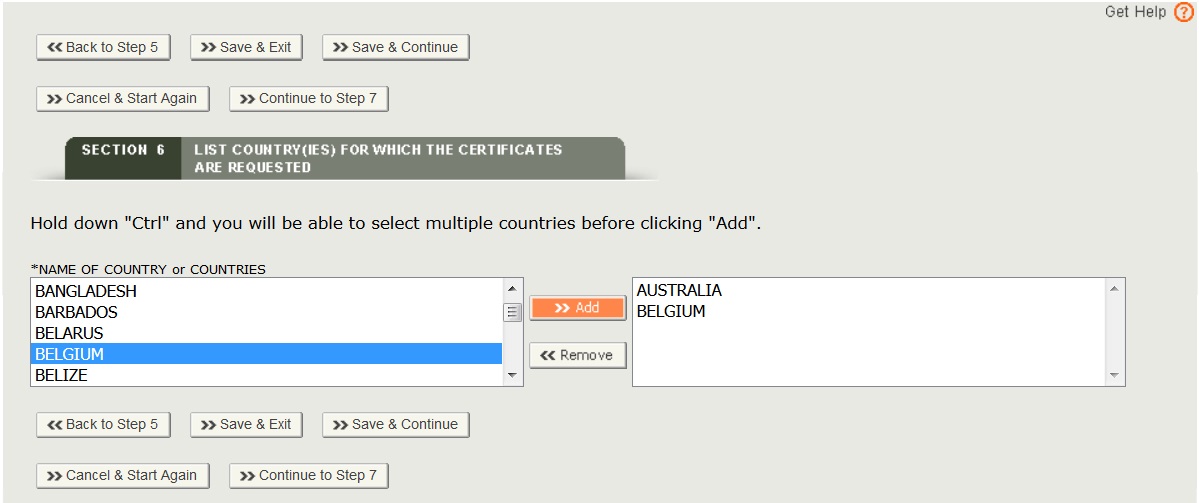
NOTE: The standard format of the printed certificate will not display an individual country name, but will state “foreign countries”. To display a specific country on the certificate, select only one country in section 6 AND indicate that country destination should be listed on the certificate in section 8.
Click “Continue to Step 7” to proceed.
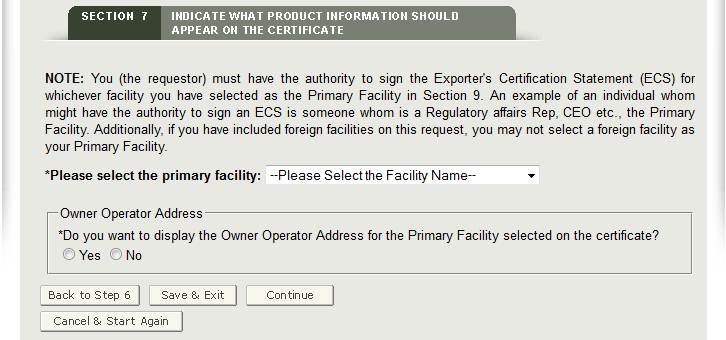
Section 7 – Indicate what product information should appear on the certificate.
Primary Facility: The primary facility is the facility which you represent. The primary facility must be a U.S establishment.
 to view a list of all U.S. facilities entered in sections 2 and 3 as shown in Figure 24 below. Select the name of the facility that you represent.
to view a list of all U.S. facilities entered in sections 2 and 3 as shown in Figure 24 below. Select the name of the facility that you represent.NOTE: By selecting the facility that you represent, CECATS will pre-populate the facility name in the Exporter’s Certification Statement which you will sign towards the end of the application.
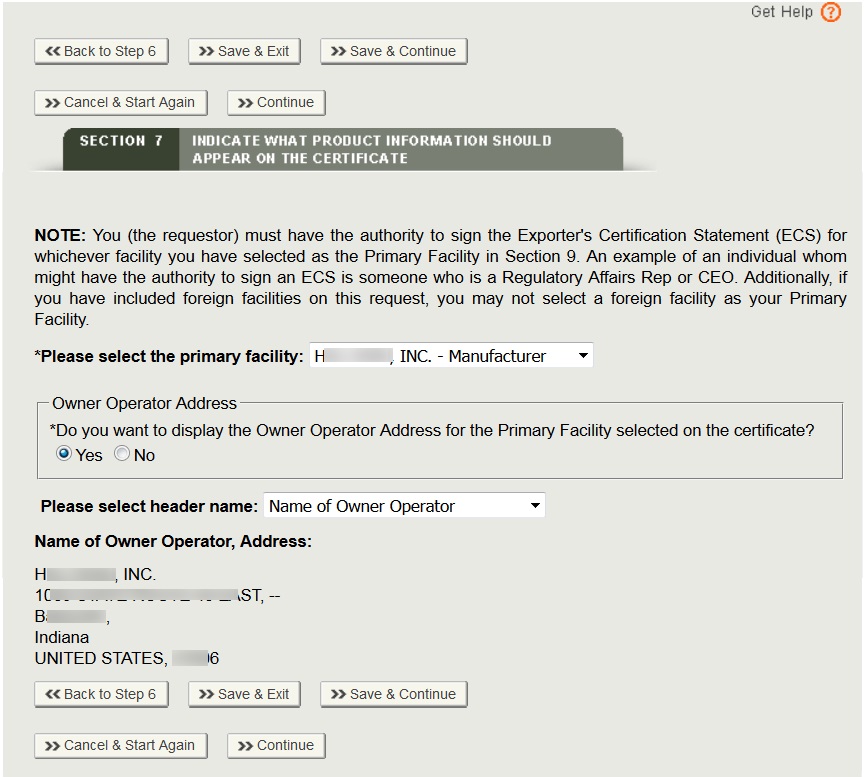
Owner Operator:
For export certificates, the Owner Operator is a facility (Corporate Headquarters) that owns one or more registered manufacturer(s) or distributor(s) in DRLM. The owner operator cannot be identified with any manufacturing role since it does not have any manufacturing role identified in DRLM. The owner operator can only be identified as Owner Operator or Corporate Headquarters. The vast majority of export certificates do not include the owner operator.
NOTE: Only the Owner Operator of the primary facility to be included on the certificate.
To display the owner operator name and address for the primary facility, click “Yes” as shown in Figure 24 below. Select the appropriate Header Name from the dropdown list and click on “Continue”.
- Name of Owner Operator: appropriate if all of the establishments on certificate have the same owner operator in DRLM
- Manufactured for Owner Operator: appropriate if all of the establishments on certificate do not have the same owner operator in DRLM
- Name of Corporate Headquarters
NOTE:If the owner operator name or address is incorrect, Save & Exit the application and update the address information in the Online Administration Account (OAA) from the FURLS homepage. When you reenter CECATS to complete the draft application, you may need to remove the primary facility from section 2 and then re-add it for the updated information to be retrieved from DRLM.
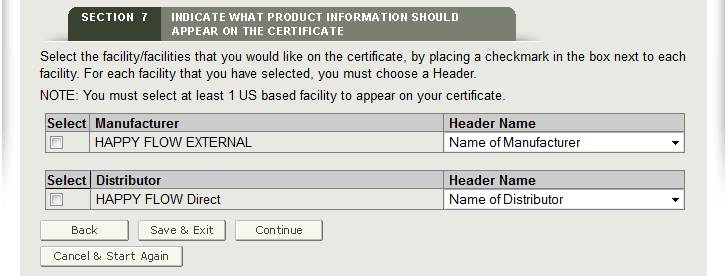
SELECTING THE FACILITY TO BE PRINTED ON THE CERTIFICATE
A list of all manufacturers and distributors entered in section 2 and 3 will appear as shown in Figure 26. Click each facility that you want to appear on the certificate.
NOTE: At least one domestic (U.S.) facility must be printed on the certificate.
Headers
For each facility, choose the Header Name that will be printed on the certificate from the Header Name dropdown list as shown in Figure 25 below. If the same header is chosen, the facilities will be grouped together under the header.
Subheaders: provide the option to identify the specific role the firm plays in regards to the products.
Optional: One or more subheaders may be selected for each facility to be printed on the certificate. Select the facility and click “Add/Remove Subheader” button as shown in Figure 26.
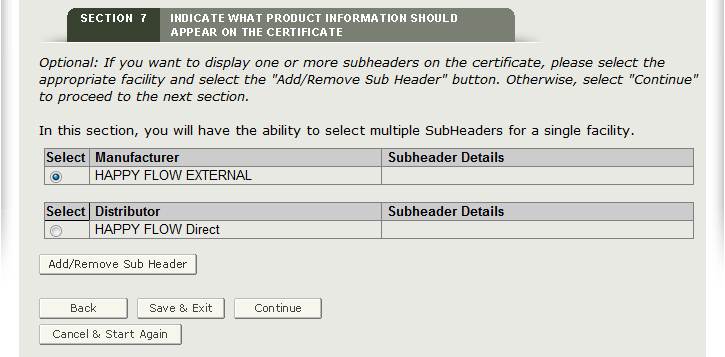
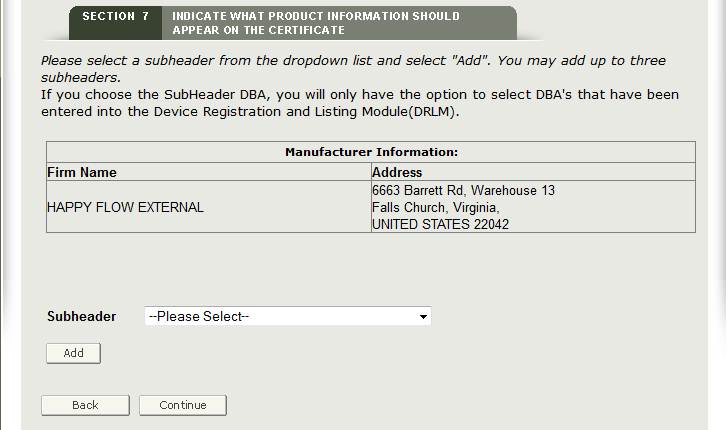
Select the appropriate Subheader from the dropdown list and then click “Add” as shown in Figure 27 below.
Subheaders are optional. If no subheader is desired, leave as “—Please select—“.
To remove the Subheader, click “Remove”.
Subheader Rules
The following describes the Subheader rules:
- “Doing Business As” (DBA)
All DBA or trade names for the establishment in DRLM will be displayed and you may choose one or more to be on the certificate. You will be given the option to choose one of the DBA names as the establishment names as well as if the Doing Business As wording is to appear.
Formatting: The DBA information will appear after the establishment name.
If CECATS does not show any DBA or trade names for the facility, the establishment registration must be updated to add a DBA name for the facility. - “Also Known As” (AKA) and “Address Also Known As” (AAKA)
Same basic function as described above for Doing Business As with Also Known As replacing the Doing Business As wording.
Formatting: The AKA information will appear after the establishment name.
Advanced function: You will have the option to enter up to three trade names with addresses under “Address Also Known As” Subheader.
Formatting: The AAKA information will appear after the establishment address.
- “Lens Finisher” or “Lens Finisher Manufacturer”: May only be chosen for the Primary Facility. When prompted, type in both the name and address of the facility. Formatting: The establishment name and address will appear after all other establishment and Subheader information.
- “a division of”
When prompted, type in the division name.
Formatting: This information will appear after the establishment name and after any DBA or AKA chosen, but before the address. - "Formerly Known As", "Manufactured for", "Made for", or "A Wholly Owned Subsidiary of":
When prompted, type in the Firm name and address, if desired.
Formatting:- Firm name only: this information will appear below the establishment name and if applicable, below rule #2 and #4.
- Firm name and address: this information will appear below the establishment name and address.
- "Previously Manufactured at":
When prompted, type in one or more Firm names and one address, if desired.
Formatting:- Firm name(s) only: this information will appear below the establishment name and if applicable, below rule #2 and #4.
- Firm name(s) and single address: this information will appear below the establishment name and address.
- "Made for":
When prompted, type in the Firm name and address.
Option: Select “by” to add the word “by” on the certificate immediately following the firm’s address.
Formatting:- “by” not included: this information will appear below the establishment name and if applicable, below rule #2 and #4.
- “by” include: the information will appear before the establishment name
Once the sub header details are entered, the system will display sub header details for each manufacturer and distributor as shown in Figure 28 below.
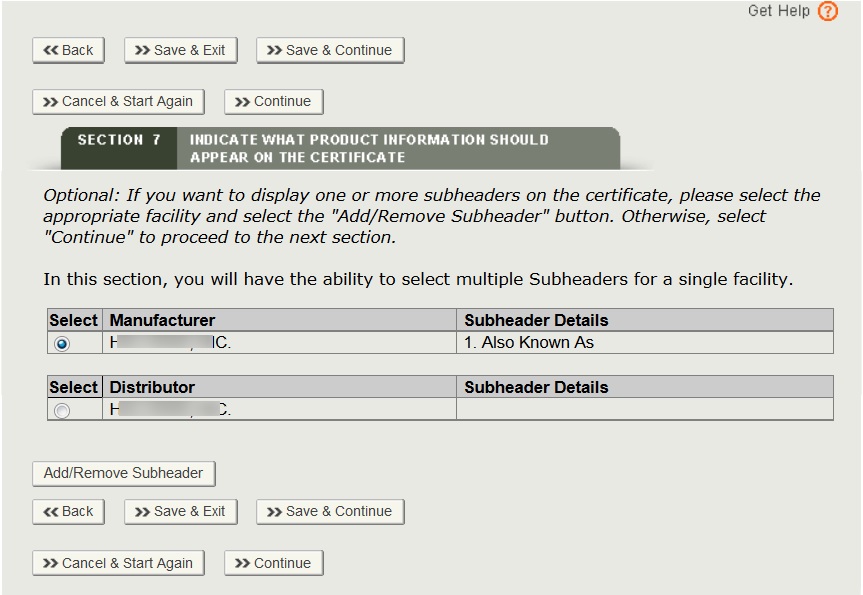
Indicate What Product Information Should Appear on the Certificate
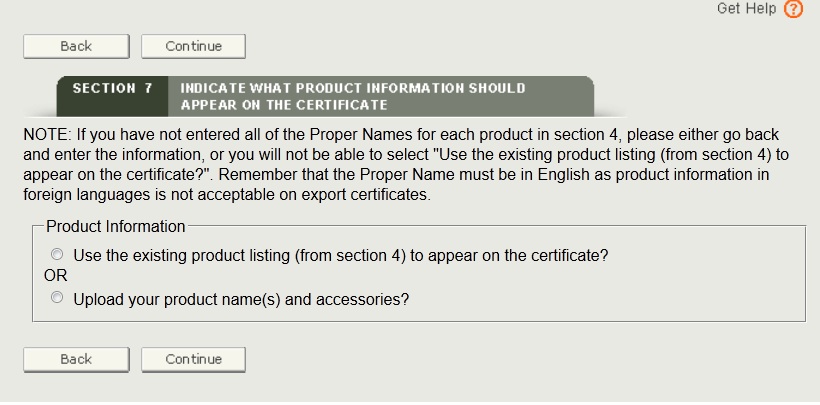
There are 2 ways to identify the names of the products to be included on the certificate (see Figure 29):
- Select “Use Existing Product Listing (from section 4) to appear on the certificate” to utilize the product’s proper name as entered in section 4. (If a proper name was not entered, please go back to section 4 and enter the proper name or choose to upload a product list.) This option is best if there are only a couple devices with short names.
- Select “Upload your product name(s) and accessories”. This option is best if there are many devices or models with long names.
OR
Upload Product List (File)
To upload your product list (File), click on the “product worksheet” hyperlink as shown in Figure 30 to download a template which is in a format that must be used to upload your products.
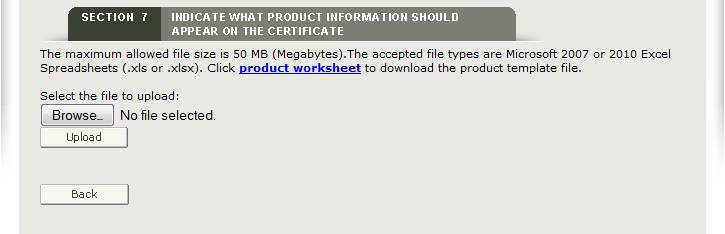
Type or copy your product list onto the template. Please read the Tips below first.
Tips for uploading products
Please adhere to the following rules or the system will not accept the upload:
- Rows 1 and 2 must not be deleted. (Product names typed on rows 1 and 2 will not appear on the certificate.)
- Please do not separate products with any blank rows. CECATS will automatically delete these blank rows on the certificate. Grouping of products can be achieved by entering a dot or dash on the line in between the group. This dot or dash will appear on the certificate.
- Please do not exceed the maximum character limit of 90 per cell.
- Once loaded into CECATS, the characters are displayed on certificates in Arial font with a size of 8.
- There is a single cell available for each product name with CECATS allowing each cell to be up to 90 characters. If you would like the appearance of columns within the one column format, a process called concatenation can be utilized. This can be accomplished using spreadsheet software such as (Microsoft Excel or Libre Office Calc) by concatenating multiple fields into a single field. Information on how to do this in Excel can be found in the Excel help. Basic concatenation instructions are provided below. For additional assistance, please contact FDA at cdrhcecats@fda.hhs.gov.
Concatenation – creating the appearance of multiple columns within a one column format.
For example, a company may store product information in a spreadsheet like this:
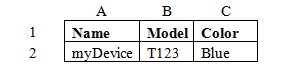
Using concatenation functions a company might use a function in another column such as
=A2&" - "&B2&" - "&C2
This takes the values found in the cells A2, B2, and C2 and puts them together in a single cell with dashes (which can be changed to any type of separating text desired). The quotes around the dashes indicate that the text (and spaces) between the quotes are actual text to be displayed rather than a reference to a cell or function in the spreadsheet. This is the result of adding the function to cell D2:

Now that the cells with various product information have been concatenated, the concatenated information needs to be moved into a spreadsheet conforming to the CECATS template spreadsheet which is a single cell. Though the concatenation function displays the concatenated text, if this cell is copied to another spreadsheet the result will likely be an error displayed in the cell. This error occurs because the displayed concatenated text is still a function which requires the cells referenced in it to exist in the proper locations. Once the function is moved to a location where those referenced cells no longer exist, the function will not behave as expected. To copy the displayed concatenated text to the CECATS template spreadsheet, copy the text but when pasting the text, the option to past only values should be used. In Microsoft Excel, the option to paste only the values that are a result of a function is found in the Paste or Paste Special options. Pasting only values will allow concatenated text to appear correctly in the CECATS template spreadsheet:

When the product list is ready and saved to your computer, click “Browse…” and navigate to the location of your file and click “Upload”. The uploaded products and accessories will be ready for grouping.
NOTE: The order of the products printed on the certificate will be the same order of the products entered in Section 4 or on the product template upload file.
Grouping a Product(s) to a Facility(ies)
Products can be grouped to one, some or all of the facilities to be printed on the certificate.
NOTE: The associations you create will impact the printout of your certificate.
NOTE: There is a checkbox located at the top-left section of the Products table. If this box is checked, all products will be selected for that particular page.
“Group All Products to All Facilities” Button
The “Group All Products to All Facilities” Button provides a quick and easy way to group all products to all facilities with a single click.
“Group” Button
Select one or multiple products from the list of products. Then select one or more facilities located to the right of the product listing. When finished, click on the "Group" button.
The system will display the grouping just created. See Figure 30 below.
The system continues to display the product listing (below the grouping). Products that have been “grouped” will now be grayed out. Continue grouping the remaining products until all products on the page have been grouped.
“Add Product” Button
You may add a new device at the beginning of the product list by clicking "Add Product". Click on “Add product”, enter the Product Name and select “Continue”. The system will display the new product at the beginning of the product list.
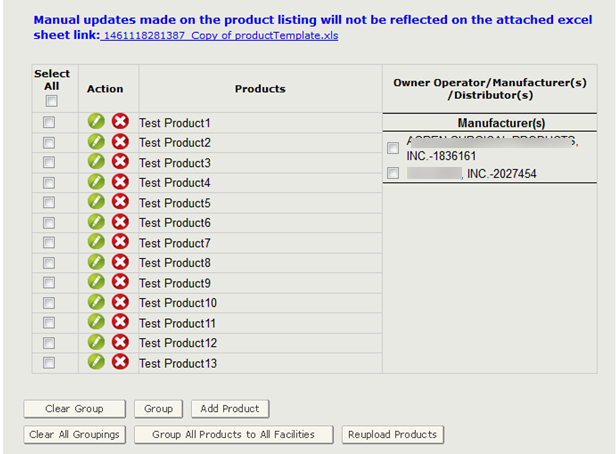
NOTE: The system will only display 30 products per page. If the product list contains more than 30 products, you must group the 30 products before moving onto the next set of products.
Once you have grouped all products on that page, click on the “Next” button to continue grouping additional products. Continue until all products on all pages have been grouped.
“Clear Group”
If an error was made when grouping, select “Clear Group” to restart grouping for that particular page.
NOTE: The “Clear Group” button will NOT affect any other pages (if more than 30 products).
“Clear All Groupings”: will remove ALL groupings from all pages. The system will provide a warning message prior to clearing all groupings. Once confirmed, you will have to start the grouping process.
“Reupload Products” if changes to the product list are required after the initial product upload, click “Reupload Products” and all products and groupings will be removed. A warning message will appear prior to clearing the product listing. Once confirmed, the system will navigate to the upload page. See Upload Product List (File) above.
“Restart Step 7” If for any reason you need to restart section 7, click on The “Restart Step 7”. A warning message will appear prior to restarting step 7. Once confirmed, the system will navigate back to the start of step 7.
Warning: All information will be deleted if you click on the “Restart Step 7” including the Primary Manufacturer, Owner Operator Address, and all identified facilities to be printed on the certificate, Header and SubHeader selections, and the product listing.
Once all products have been grouped to a facility or facilities, the system will display the product groupings for review as shown in Figure 32 below
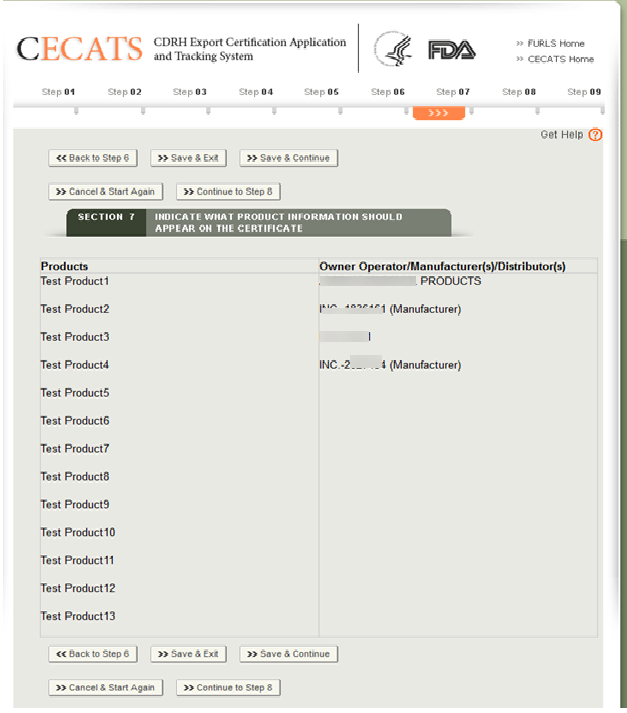
Click “Continue to Step 8”.
Section 8 – Should the country destination be listed on the certificate?
Unless otherwise indicated in this section, the certificate will be state “into foreign countries.” Sometimes a specific destination country name is desired. To have a specific country appear:
- Identify only the specific country in section 6.
- Select “Yes” to the “Should the country destination be listed on the certificate” in section 8.
NOTE: If this option is greyed out and cannot be chose, most often it is because more than one country was selected in section 6.
Enter the total number of certificates requested. See Figure 33 below.

Prior to Step 9, the system will calculate the total fee as shown in Figure 34.
NOTE: The FDA will invoice your firm quarterly for all certificates issued during the quarter.
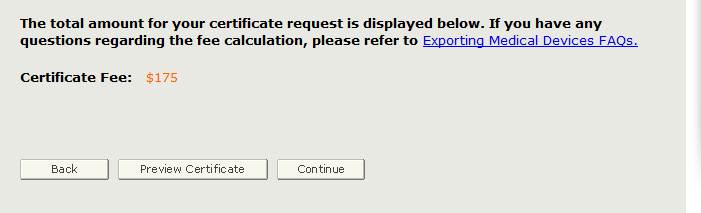
PREVIEW THE CERTIFICATE!!! To ensure that the facility name and address is appropriate, headers, subheaders, product list and groupings appear as preferred, click “Preview Certificate”. If any changes are required, return to the appropriate section and update prior to continuing onto Section 9.
NOTE: The order of the facilities on the final certificates may not be the same as the sample.
Section 9 – Exporter’s Certification Statement (ECS)
The Exporter’s Certification Statement (ECS) acknowledges that you, th responsible official or designee, certify that the facility(s) and the products identified on the Supplemental Information are to the best of your knowledge in substantial compliance with the Federal Food, Drug, and Cosmetic Act (the Act) and all applicable or pertinent regulations.
In this section, the system auto-populates the primary facility field based on the selection you made in section 7. You must select the “I Agree” button located at the bottom of this section and enter your name and title. You will not be able to continue with the application until these fields have been completed. See Figure 35 below:
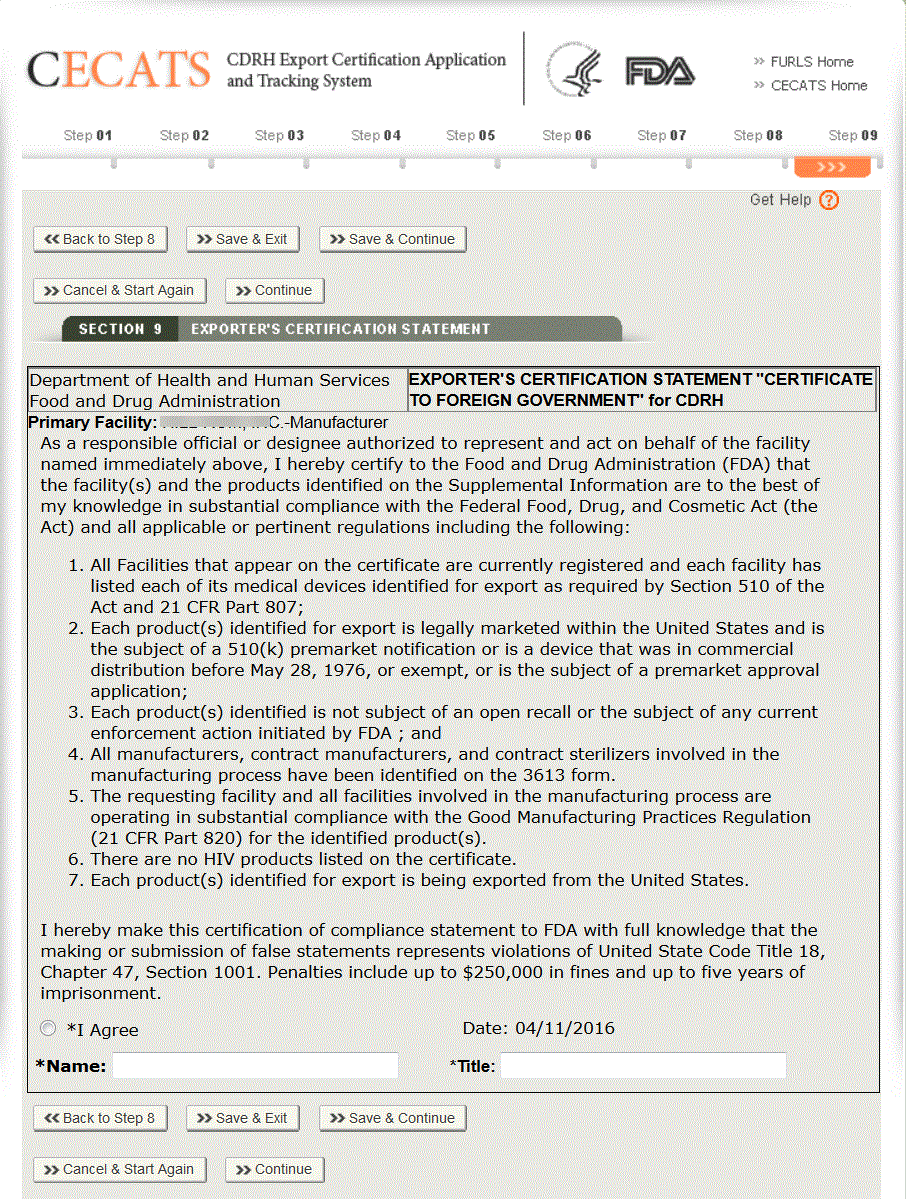
Once these fields are completed, click “Continue”.
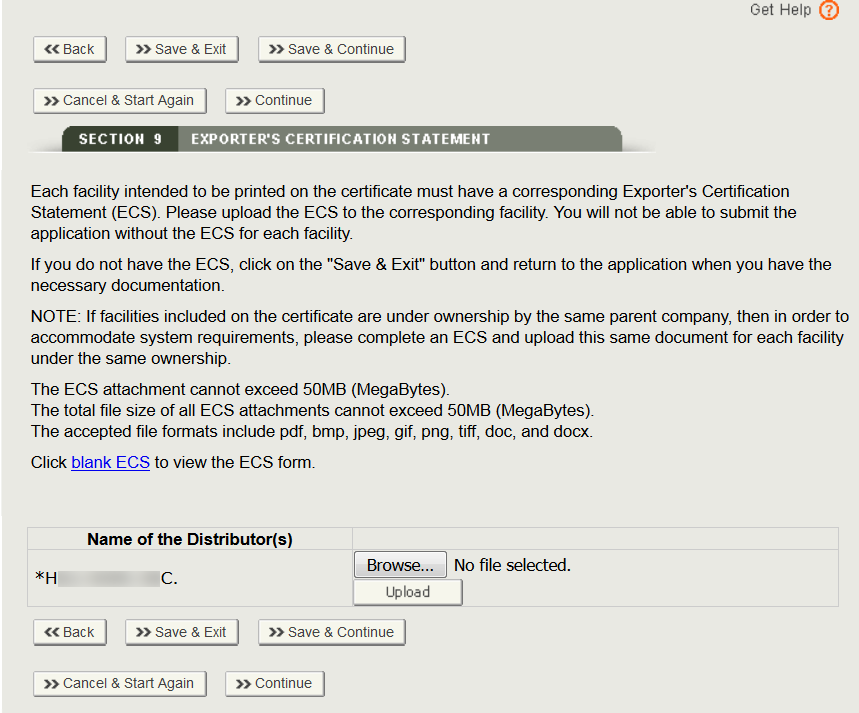
Every establishment listed on the certificate must provide a signed ECS. If the establishments are related, i.e. have the same owner operator in DRLM, the same signed ECS can be upload for all of the related establishments.
NOTE: The following is the link to the FDA 3613 CFG form including the ECS document: http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM052378.pdf
Where prompted, upload a signed ECS document for each facility by clicking on the Browse button next to the facility, select the file, and click on “Upload” in section 7 as shown in Figure 36 below:
The uploaded documents will be displayed as shown in Figure 37 below. To remove a document, simply click “Remove”.
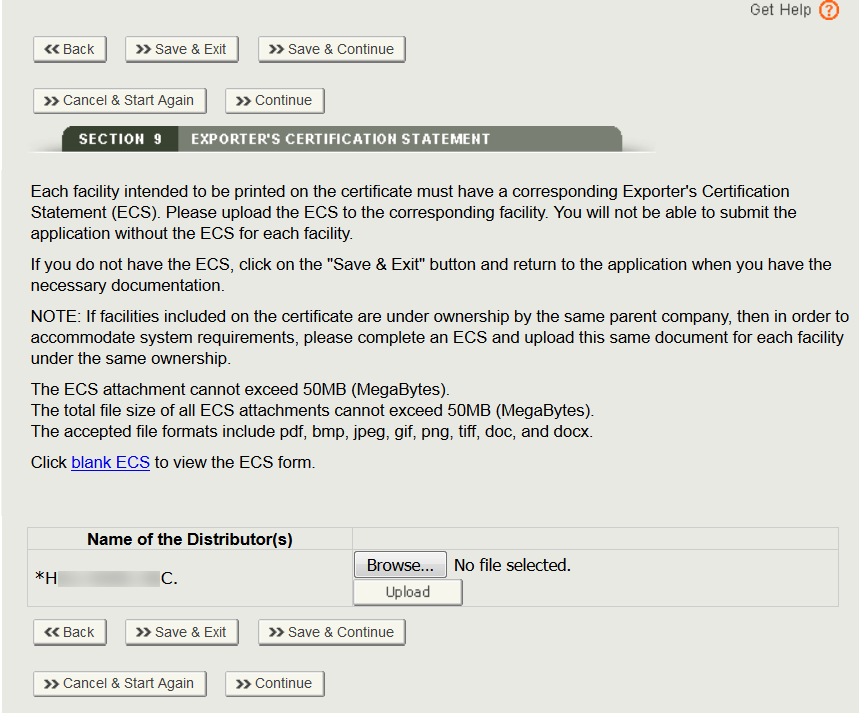
Perform the same steps for any other facility. Once all the ECS documents have been uploaded, click “Continue” to navigate to the final review page.
NOTE: The following are limitations for ECS Upload:
- The total file size of all ECS attachments cannot exceed 50 MB.
- The accepted file formats include the following: pdf, bmp, jpeg, gif, png, tiff, doc, and docx.
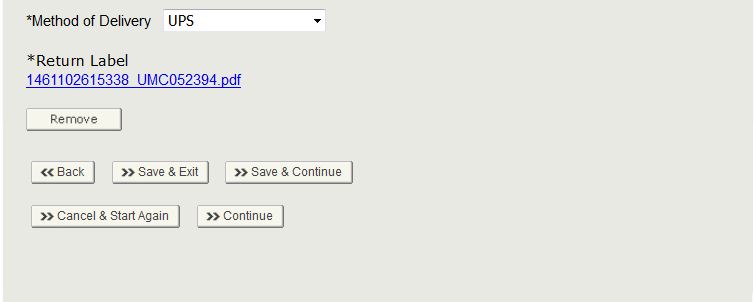
Method of Delivery
You must select the method of delivery. Select the carrier and attach a complete return label as shown below in Figure 38.
Final Review Screen
The system will display the entire application broken out by section (See Figure 39 below). You may choose to modify a section by selecting the “Edit” button next to the section to be updated. The system will re-display the data entry screen corresponding to your chosen section. You may make changes as needed.
At this time, you may preview your certificate in order to make sure that your product information, manufacturer/distributor information is displayed to your satisfaction on the certificate and attachment pages, by clicking the button “Preview Certificate”. Please see Figure 39 below. Through clicking this button, you also will be able to see the approximate number of certificate and attachment pages for your completed certificate in order to guesstimate cost information.
NOTE: Your submission is not complete until you click the “Submit”.
This will be your final opportunity to make edits to some sections, so please review your completed application carefully.
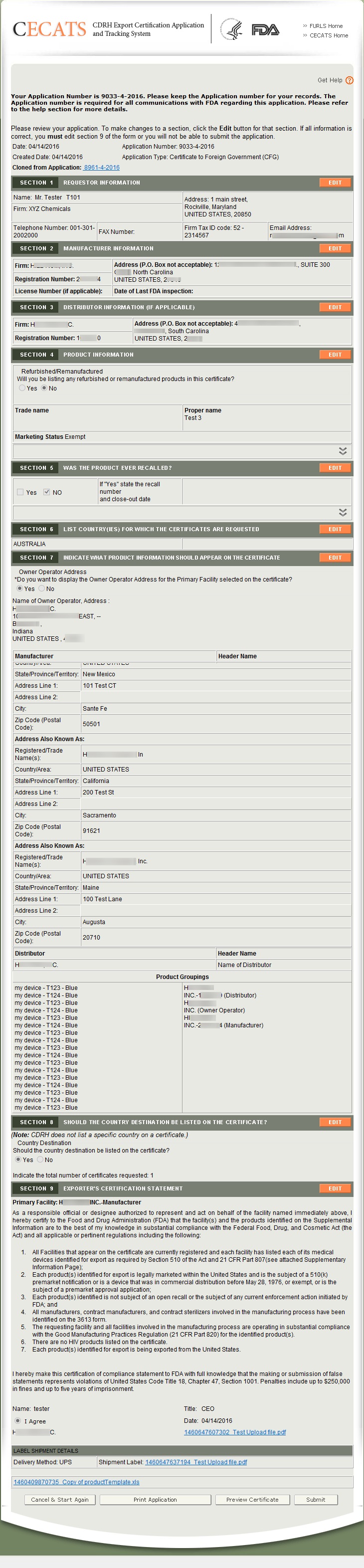
The system will display the entire application broken out by section (See Figure 39 above). You may choose to modify a section by selecting “Edit” next to the section to be updated. The system will re-display the data entry screen corresponding to your chosen section. You may make changes as needed.
NOTE: The system displays one entry in each section. If you entered multiple entries in a section, (i.e. Multiple manufacturers or distributors in section 2 or 3),
 to expand the list for that section.
to expand the list for that section.Click “Preview Certificate” to ensure that your product information, manufacturer/distributor information is displayed to your satisfaction on the certificate and attachment pages. Please see Figure 39 above.
This will be your final opportunity to make edits to some sections, so please review your completed application carefully.
NOTE: Your submission is not complete until you click “Submit”.
You may choose to print your application prior to submission. Select “Print Application” located at the bottom of the review page. A new browser window will open which will allow you to print the application. When you are finished, close the browser window in order to return to the CECATS application.
Note: If you have not previously saved the application, the application number will not appear on the printed application. You might want to wait until you Save or Submit the application so you have the application number for reference.
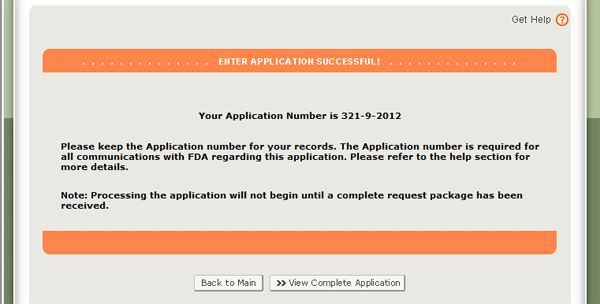
When your application is ready for submission, click “Submit” also located at the bottom of the review page. The system will display a message that your application was successfully submitted as shown in Figure 40 below. An application number will be displayed and the opportunity to print a copy of the application is available. Please save the application number for future reference. The application number will be required to check the status of your application. You will also receive an email confirmation that your application has been successfully received along with the application number.
Clone An Application
The cloning feature allows a user to copy any submitted application including the CFG, 801(e)(1), and the 802. Once cloned, the user will have the ability to update any or all sections of the application prior to submission.
NOTE:
- Applications in any status except “Draft” status can be cloned. This includes any application in a “Rejected” or “Cancelled” status.
- An application cannot be submitted without updating the Exporter’s Certification Statement.
To clone an application,
- Select "Enter New Application" from the main menu (Refer to Figure 2).
- Select the application to be cloned
- Click “Clone Application”. All sections will be editable. (Refer to Figure 39) You must edit section 9 to sign the application and upload a shipping label and any ECSs required.
NOTE: If your list of applications becomes too large, the Search function is viable option to quickly locate a specific application. - When your application is ready for submission, click “Submit”.
NOTE: Remember to update section 9 or you will not be able to submit the application.
Once submitted, the application will be assigned an application number and you will receive an email stating your application was successfully submitted (Refer to Figure 41). Please save this number for future reference. The application number will be required to check the status of your application.
